Abstract
Introduction Next-Generation Sequencing (NGS) implementation to perform accurate diagnosis in acute myeloid leukemia (AML) represents a major challenge for molecular diagnostic laboratories in terms of the specialization, cost and logistical support. The PETHEMA group established a diagnostic network of 7 reference laboratories to provide standardized NGS studies for AML patients, performing cross-validation (CV) rounds to ensure the quality of the studies and regular updating of gene panels. We report the CV rounds of the diagnostic platform and the clinical validation of the new genomic classification which proposes unified framework for disease classification and risk-stratification in AML based on cytogenetic and 32 genes (Tazi et al. 2022).
Methods CV rounds were performed by exchanging control samples among reference laboratories every 9-12 months. In the last CV round, four control samples harboring 34 variants were distributed (21 variants with VAF>5% and 13 with VAF<5%). NGS analyses were performed in 1631 samples collected from 1471 patients enrolled in NGS-AML project (NCT03311815). Genomic classification and risk stratification was validated in 954 patients treated with intensive (n=568) or non-intensive therapy (n=386) with available clinical, cytogenetic and molecular data. An additional cohort of 1225 samples was included for molecular analysis.
Results The error rate (ER) in variants with VAF>5% was decreasing in the successive CV rounds (1st: 39%, 2nd: 14.4% and 3rd: 4.76%). However, in variants with VAF <5%, ER was raised (2nd: 28.6% and 3st: 59.62%). Consequently, the diagnostic platform decided to maintain the cut-off of VAF>5% to report clinically relevant variants.
In the global cohort, 7768 variants were detected. 96.5% of samples showed at least one mutation, with a mean of 3.1 mutations/sample (range 0-13). FLT3 (24.6%), DNMT3A (24.3%), NPM1 (22.4%) and TET2 (21.6%) were the most frequently mutated genes. Our series also reflected different mutational profile according to disease status (DNMT3A, IDH1, KRAS, NPM1, NRAS, RUNX1 and TP53), age (ASXL1, DNMT3A, EZH2, FLT3, IDH2, NPM1, RUNX1, SF3B1, SRSF2, TP53 and U2AF1) and sex (ASXL1, DNMT3A, EZH2, FLT3, NPM1, RUNX1, SRSF2, and U2AF1); and revealed co-occurring mutations and exclusivity gene patterns (Table 1).
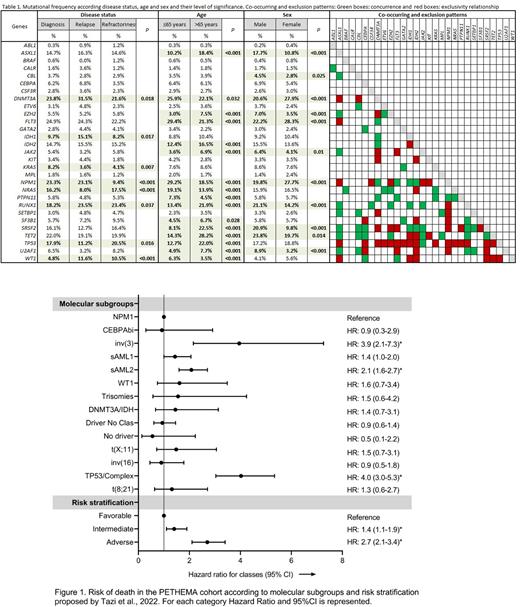
According to genomic classification, the PETHEMA cohort was categorized in 16 molecular classes which encompass established WHO2016 entities (33.5%) [inv(16), t(8;21), NPM1, CEBPAbi, t(11;x), t(6;9) and inv(3)] and novel entities: "sAML1" (8.7%) [Single mutation in: SRSF2, SF3B1, U2AF1, ASXL1, EZH2, RUNX1 and SETBP1], "TP53/complex karyotype" (19.2%), "sAML2" (25.4%) [More than one mutated sAML1 gene], "driver mutations but no defining class" (8.5%), "DNMT3A/IDH1-2" (1.8%), "WT1" (1.4%), trisomies (0.73%) and "No events" (0.94%). These molecular subgroups have also been associated with different prognostic values: "TP53/Complex karyotype" [4.2 months (95%CI 2.9-5.5)], inv(3) [4.9 months (95%CI 0.8-9.1)] and "sAML2" [11.87 months (95%CI 9.7-14.0)] showed the lower values of OS, while patients classified in the "No events" and "inv(16)" (Median OS not reached at 33 and 42 months) and "NPM1" [28.97 months (95%CI 19.9-38.0)] had the best outcomes. Figure 1.
Integrated risk score based on cytogenetic and 32 genes was assessed in the PETHEMA "real-life" cohort: 23.5% of patients were classified in the favorable risk group [OS 29.23 months (95%CI 18.40-40.06)], 27.5% in the intermediate [18.77 months (95%CI 14.62-22.92)] and 49.1% in the adverse risk group [9.37 months (95%CI 7.76-10.98)]; (P<0.001). HR are shown in figure 1. Finally, we identified a dismal prognosis and higher risk of death in older patients compared to younger ones for all risk groups: Favorable: 4.5 (95%CI 2.68-7.56; P<0.001), intermediate: 1.93 (95%CI 1.33-2.79; P<0.01) and adverse: 2.63 (95%CI 2.01-3.46; P<0.001).
Conclusions We show the experience of the PETHEMA diagnostic network in standardized NGS studies in terms of quality metrics and variant reporting criteria. Based on the current landscape of AML, these strategies ensure technical quality and equity in access to NGS studies. Furthermore, this cooperative work allows the assessment of new molecular subgroups in "real-life" cohorts as well as the application of the novel risk classifications.
Partially funded by BMS/Celgene. PI18/01340, PI19/00730, FI19/00059
Disclosures
Ayala:Altum Sequencing: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Celgene: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bilbao:Astellas: Other: Speakers. Bergua Burgués:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Alonso Dominguez:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding. García-Sanz:Janssen: Honoraria, Other: Travel support, Research Funding; BeiGene: Honoraria, Other: Travel Support; Gilead: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Amgen: Honoraria; Takeda: Honoraria, Research Funding; GSK: Honoraria, Other: Travel Support; Astra Zeneca: Honoraria; In Vivo Scribe: Patents & Royalties: Indirect perception, Euroclonality primers; Novartis: Honoraria, Research Funding. Perez-Simon:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ABBVIE: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; GILEAD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; JAZZ: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ALEXION: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; PFIZER: Research Funding. Gómez-Casares:Novartis: Speakers Bureau; Pfizer: Speakers Bureau; BMS: Speakers Bureau. Montesinos:KURA ONCOLOGY: Consultancy; ABBVIE: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; NOVARTIS: Consultancy, Research Funding, Speakers Bureau; JAZZPHARMA: Consultancy, Research Funding, Speakers Bureau; BEIGENE: Consultancy; ASTELLAS: Consultancy, Speakers Bureau; PFIZER: Consultancy, Research Funding, Speakers Bureau; INCYTE: Consultancy; TAKEDA: Consultancy, Research Funding; RYVU: Consultancy; NERVIANO: Consultancy; OTSUKA: Consultancy; GILEAD: Consultancy, Speakers Bureau; MENARINI/STEMLINE: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal